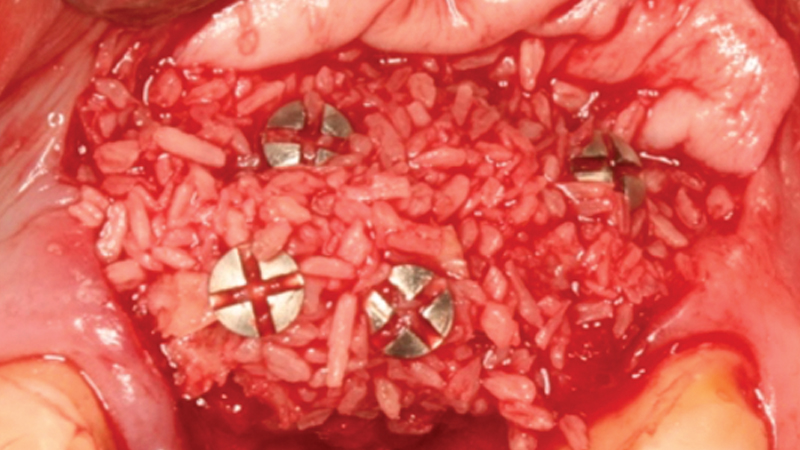
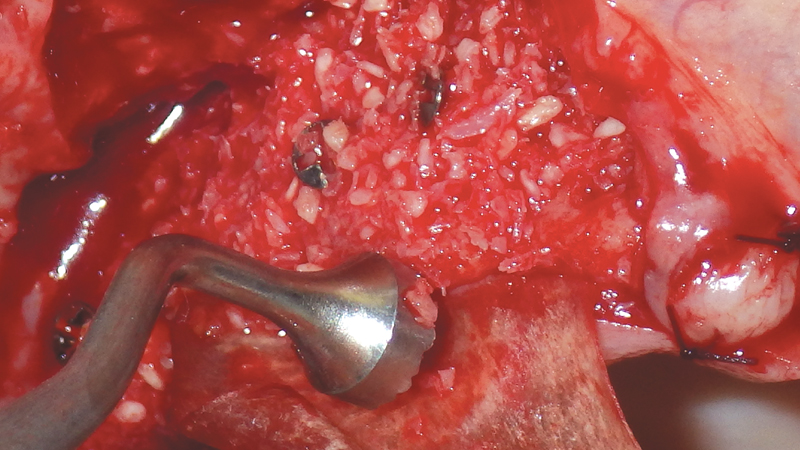
- Streamlines the bone grafting process by providing a simplified assortment at great prices
- Bone grafting materials, instruments, membranes and wound dressing, all from a single convenient supplier
- FDA-cleared and naturally sourced
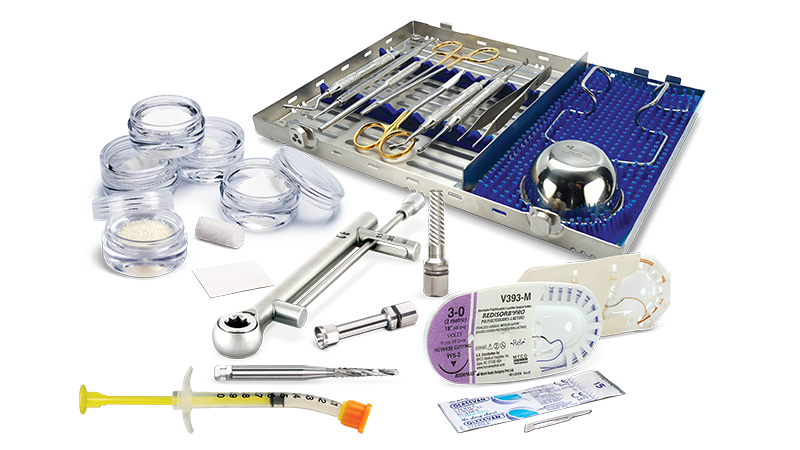

Newport Surgical™ Bone Grafting Solutions
The Newport Surgical™ line of bone grafting solutions features a simplified set of high-quality instruments and materials to help dentists efficiently and reliably perform the majority of bone grafting procedures with confidence.
Simplifying the Art of Regenerative Science
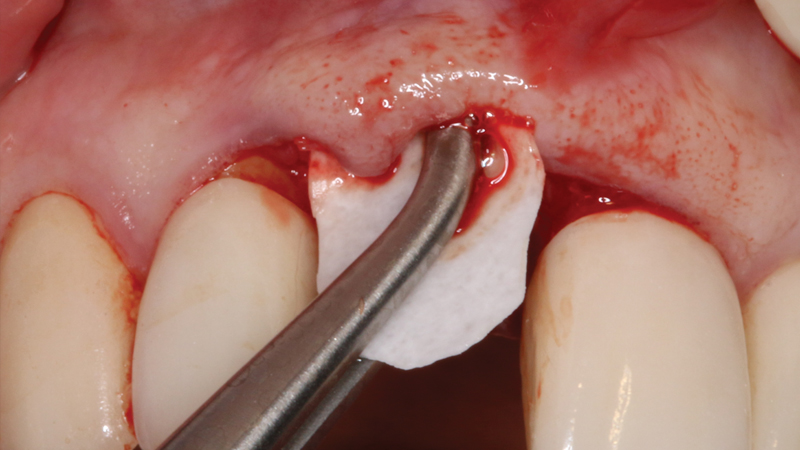
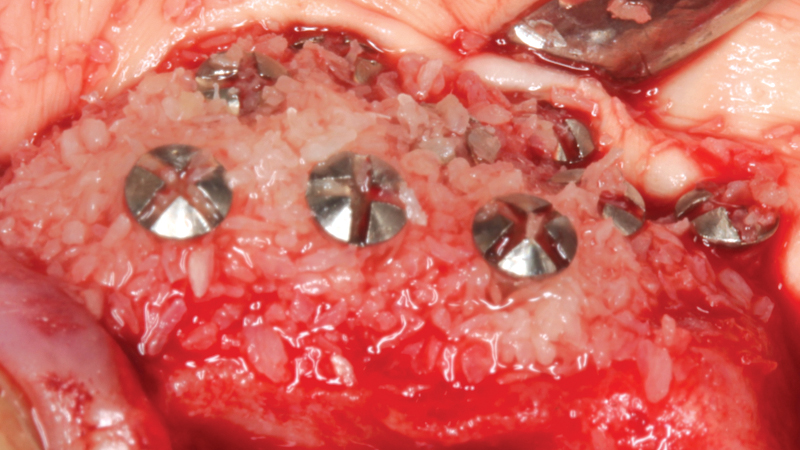
Helping to maximize the body’s bone healing and regeneration process, the Newport Surgical line of bone grafting materials and instruments provides clinicians with a simplified buying experience, unparalleled value, and the confidence to efficiently and reliably perform the majority of bone grafting procedures. These include extraction socket regeneration, ridge augmentation, sinus floor elevation, periodontal reconstruction and peri-implant management.
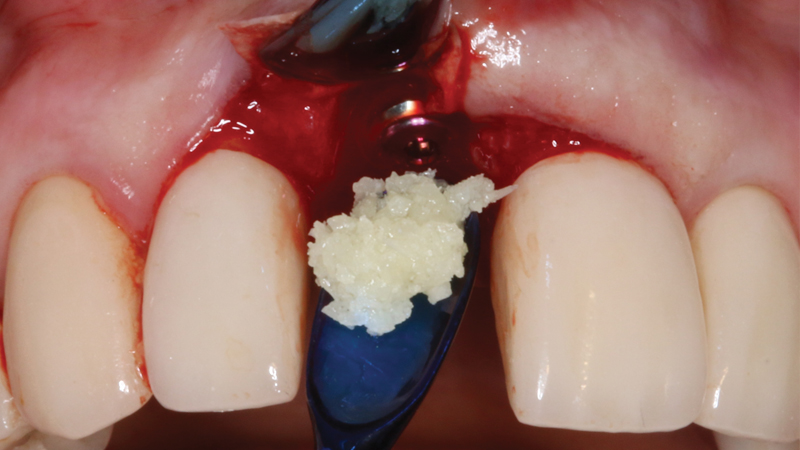
For bone grafting applications, Newport Surgical offers Newport Biologics Mineralized Cortico/Cancellous Allograft Blend and Newport Biologics Bone Graft Putty Mineral-Collagen Composite. Both materials serve to establish osteoconductive matrices that promote bone regeneration. Prepackaged in a syringe that is ready to use after a simple rehydration procedure, RAPTOS® Cortico-Cancellous Blend offers a convenient means of grafting sites to form an osteoconductive scaffold for predictable bone regeneration.
Newport Surgical also offers a two-in-one resorbable plug, called the OsteoGen® Plug. This product serves as both the wound dressing and bone grafting material for ridge preservation and socket regeneration.
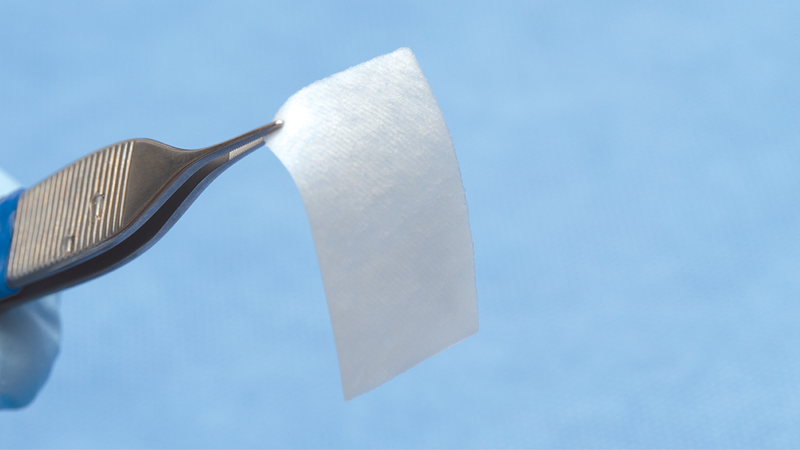
Manufactured from highly purified porcine tissue, Newport Surgical Collagen Membranes are available with two different resorption schedules: 3–4 months and 4–6 months. Resorbable Collagen Plugs are also available for wound dressing purposes and the management of extraction sites.
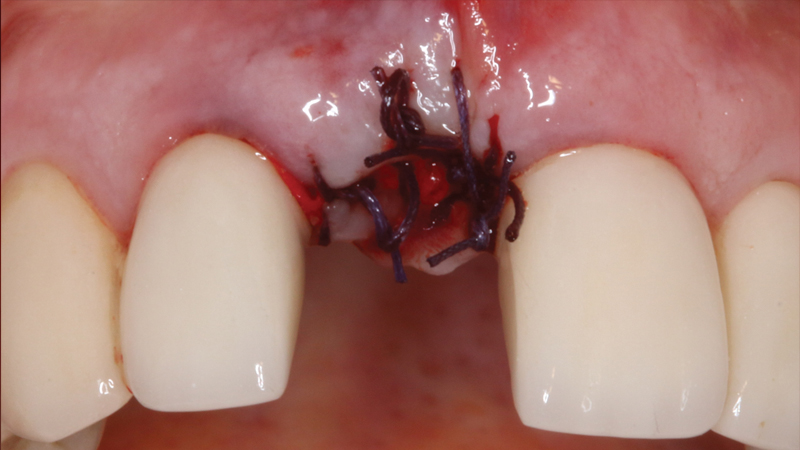
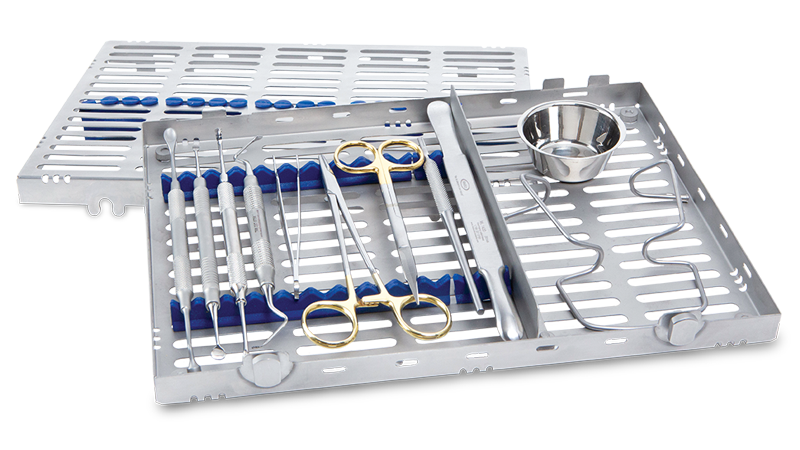
The Newport Surgical Implant and Bone Grafting Instrument Kit includes the primary tools you need to perform most bone grafting procedures in your practice, including 12 durable instruments made from high-quality German steel. RELI® REDISORB® PRO Sutures are resorbable, quick and simple to access without knots or tangles, and available in polyglycolic acid (PGA) and chromic gut with a 3/8 reverse cutting needle. Available in size #15, GLASSVAN® Surgical Blades feature newly refined cutting edges that improve the sharpness of the incision and reduce drag.
RAPTOS is a registered trademark of Citagenix Inc. OsteoGen is a registered trademark of IMPLADENT, LTD. RELI, REDISORB and GLASSVAN are registered trademarks of Myco Medical Supplies, Inc.
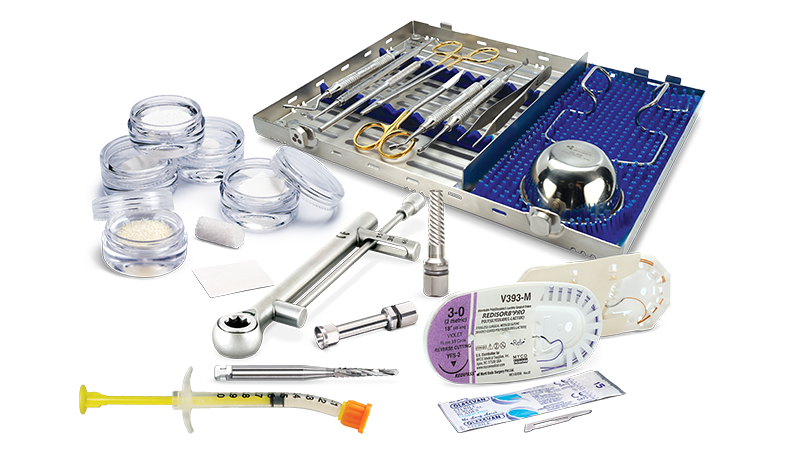
Bone Grafting Solutions Meet Clinical Needs
Resultant bone resorption after tooth removal can be approximately 1.5–2 mm vertically and 3.8 mm in the horizontal plane within six months.1
Mineralized bone allograft has been shown to be a better scaffold for osteoconduction than demineralized bone allograft, allowing for superior space maintenance.2
Ideal Characteristics of Membrane Material
- Tissue compatibility
- Space maintenance
- Stabilization of the blood clot
- Cell occlusiveness
- Mechanical strength
- Predictable resportion rate
- Easy to modify and manipulate
Source: Resnik RR. Use of barrier membranes in implant dentistry. Chairside. 2018;13(1):48.
Ideal Characteristics of Bone Graft Material
- Biocompatibility
- Bioactive to promote cell differentiation and proliferation
- Low incidence of infection
- Nontoxic and non-immunogenic
- Ability to maintain space and volume overtime
- Ability to be replaced entirely with new bone growth
- Resorption rate to coincide with bone formation
Source: Resnik RR. Bone substitutes in oral implantology. Chairside. 2018;12(3):84.

Clinical Studies
Predictable Solution for Implant Treatment
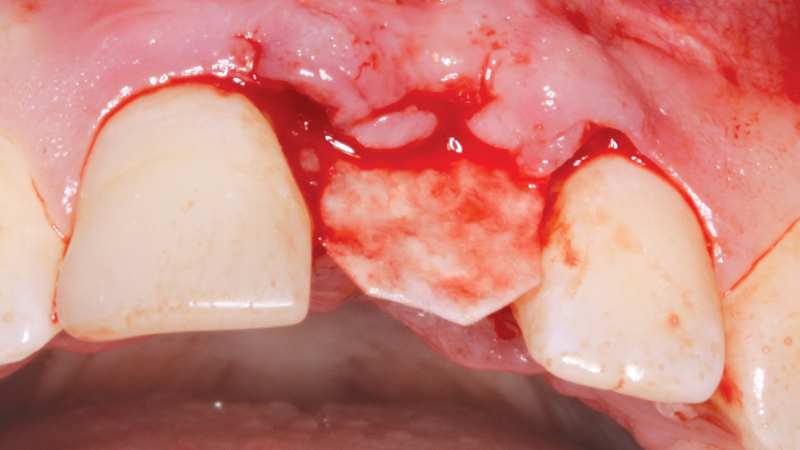
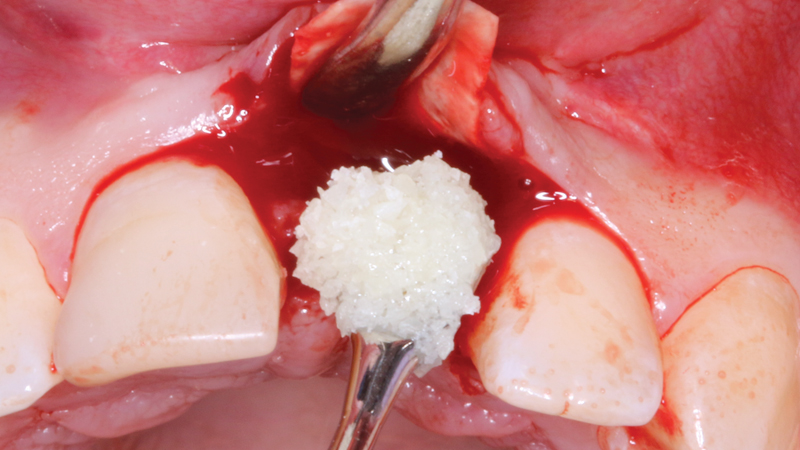
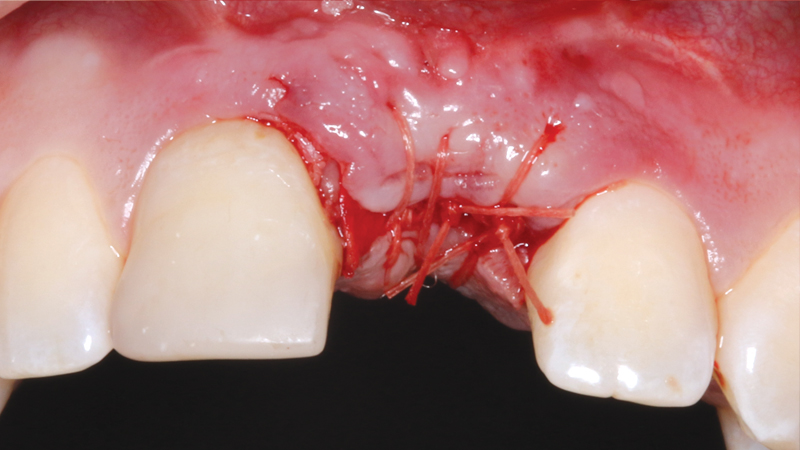
Newport Surgical Resorbable Collagen Membrane 3-4 and Newport Biologics Mineralized Cortico/Cancellous Allograft Blend provide a predictable regenerative solution for implant treatment.
– Clinical dentistry by Timothy F. Kosinski, DDS, MAGD
- Slide 1
- Slide 2
- Slide 3
Newport Surgical offers the core products necessary for virtually all implant and GBR (guided bone regeneration) needs. The Mineralized Cortico/Cancellous Allograft Blend is available in convenient sizes suitable for a simple socket graft all the way up to full-arch procedures or sinus lifts.
- Slide 1
- Slide 2
Indications
Newport Biologics Mineralized Cortico/Cancellous Allograft Blend
Newport Biologics Mineralized Cortico/Cancellous Allograft Blend is used in dental bone grafting procedures where a human allograft is appropriate. Newport Surgical allografts are declared safe for transplant. Donor eligibility (screening and testing) is performed in accordance with AATB Standards and FDA regulations.
RAPTOS Cortico-Cancellous Blend in a Syringe
RAPTOS Cortico-Cancellous Blend is a 100% human allograft bone product provided in a specially designed syringe that enables rehydration in the syringe with saline or patient’s blood. Composed of bone from donors screened and tested in accordance with FDA regulations, this mineralized allograft blend is used in grafting procedures for extraction sockets, bony defects, immediate implant sites, and a range of other clinical applications.
Newport Biologics Bone Graft Putty Mineral-Collagen Composite
Newport Biologics Bone Graft Putty Mineral-Collagen Composite is a sterile, biocompatible synthetic calcium phosphate mineral plus collagen for use in periodontal, oral and maxillofacial surgery.
Resorbable Collagen Membrane 3-4
Resorbable Collagen Membrane 3-4 is a bioresorbable, implantable collagen material that is intended for use in oral surgical procedures as a resorbable membrane material for use in the following: simultaneous use of guided bone regeneration (GBR)-membrane and implants; augmentation around implants placed in immediate extraction sockets; augmentation around implants placed in delayed extraction sockets; localized ridge augmentation for later implantation; alveolar ridge reconstruction for prosthetic treatment; filling of bone defects after root resection, cystectomy or removal of retained teeth; guided bone regeneration in dehiscence defects; and guided tissue regeneration procedures in periodontal defects.
Resorbable Collagen Membrane 4-6
Resorbable Collagen Membrane 4-6 is a bioresorbable, implantable collagen material that is intended for use in oral surgery procedures as a material for placement in the area of dental implant, bone defect or ridge reconstruction to aid in wound healing.
Resorbable Collagen Plug
Resorbable Collagen Plug is indicated for the management of oral wounds and sores, including denture sores, oral ulcers (non-infected or viral), periodontal surgical wounds, suture sites, burns, extraction sites, surgical wounds, and traumatic wounds.
OsteoGen Plug
OsteoGen non-ceramic crystals resorb over 3-5 months and serve as both the wound dressing and bone grafting material for ridge preservation and socket regeneration.
Material Composition
- Newport Biologics Mineralized Cortico/Cancellous Allograft Blend: Mix of cortical and cancellous particulates
- RAPTOS Cortico-Cancellous Blend in a Syringe
- Newport Biologics Bone Graft Putty Mineral-Collagen Composite: Carbonate apatite plus bovine type I collagen
- Resorbable Collagen Membrane 3-4: Purified porcine peritoneum tissue
- Resorbable Collagen Membrane 4-6: Highly purified type I collagen derived from porcine tendon
- Resorbable Collagen Plug: Collagen derived from bovine dermis
- OsteoGen Plug: Type I collagen provides scaffolding for keratinized tissue to develop